What causes calciphylaxis?
Calciphylaxis, also known as calcific uremic arteriopathy, is a rare disease with a high mortality rate that mostly affects people with end-stage kidney disease (ESKD). It is characterized by painful sores and wounds that are hard to heal.
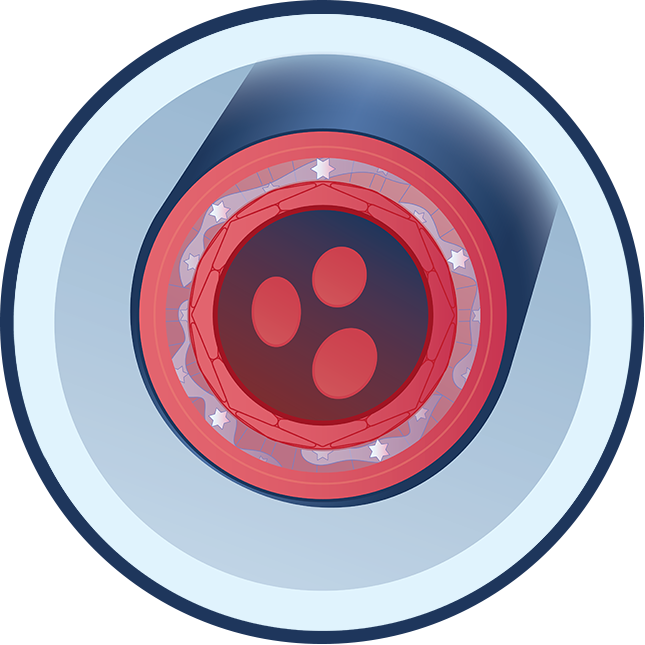
In calciphylaxis, there is an imbalance in the molecules that control mineralization. One of these critical molecules is PPi. Low PPi contributes to hydroxyapatite crystal buildup inside the small blood vessels (arterioles) near the skin and adipose tissue. Overgrowth of smooth muscle cells due to low adenosine can further block the blood vessels. These issues lead to poor blood flow, blood clots, painful skin ulcers, serious infections, and in many cases, death. Initial skin lesions typically present as extremely painful plaques and nodules, and progress to severe ulcers and tissue death. Approximately 50% of calciphylaxis patients die within a year of diagnosis.
There are currently no approved therapies for calciphylaxis.
Estimated incidence
3.5: 1,000
dialysis patients
Living with calciphylaxis
Poor Blood Flow
Hydroxyapatite crystal deposits form in blood vessels and block blood flow, leading to sores where skin and tissue break down and die.
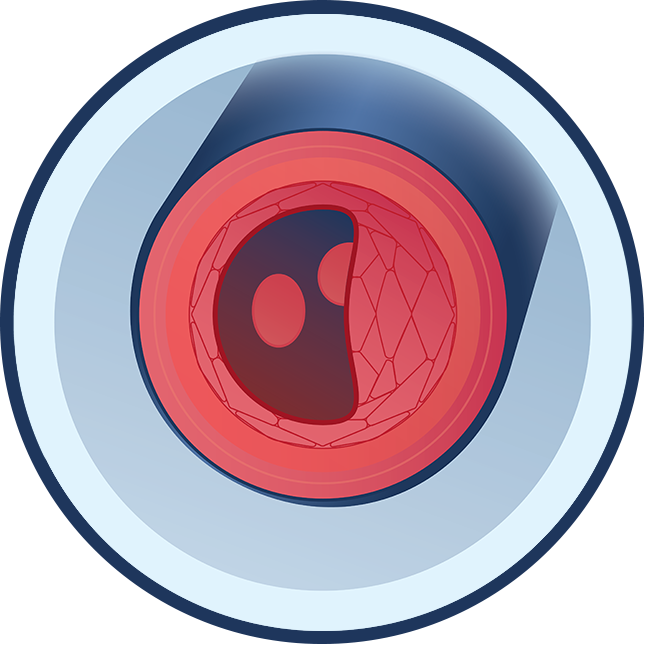
Blood Clots
Recent studies show that most people with calciphylaxis have abnormalities in blood-clotting factors. These abnormalities can lead to small blood clots forming more often than they normally would. Hydroxyapatite crystals are deposited in the smallest parts of the arteries, which also leads to the formation of blood clots.

Skin Ulcers
Deep, painful lumps ulcerate and create open sores that fail to heal. These ulcers often occur on the skin of the abdomen and thighs but can appear anywhere on the body.
Serious Infections
Serious infections occur due to wounds on the skin that spread and do not heal, which can lead to major complications including sepsis (blood infection), the most common cause of death in people with calciphylaxis.