What causes ENPP1 Deficiency?
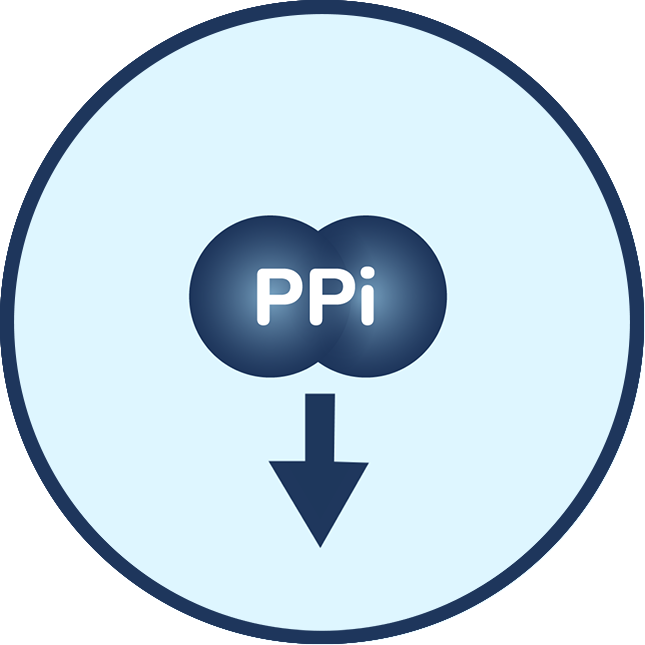
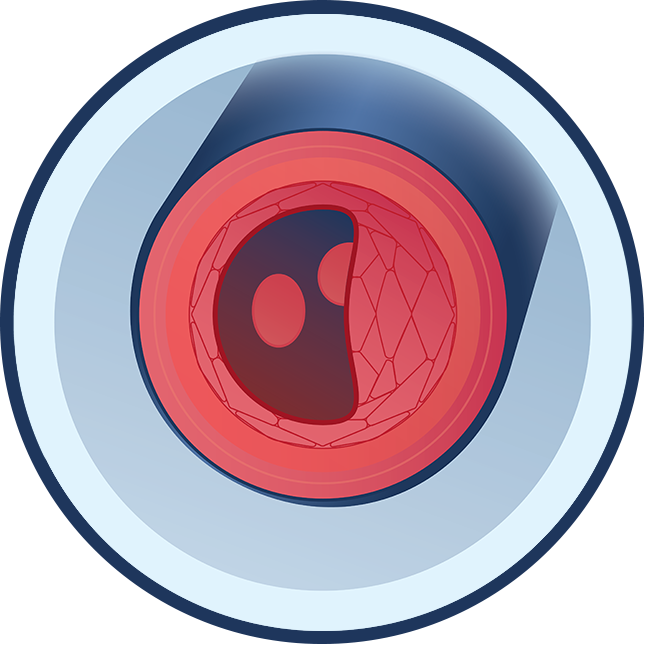
People with ENPP1 Deficiency have one or two ENPP1 gene mutations, which may cause ENPP1 enzyme activity to be reduced or even absent. ENPP1 is important in regulating the levels of two key molecules: PPi and adenosine. In ENPP1 Deficiency, the body has trouble with bone formation due to low levels of PPi and maintaining blood vessel health due to low levels of PPi and adenosine.
There are currently no approved therapies for ENPP1 Deficiency.